Manufacturing
Manufacturing / Packaging / Analysis
Meiji Seika Pharmatech Co., Ltd. produces a variety of antibacterial and generic drugs in solid dosage forms such as tablets and fine granules, as well as injectable drugs such as vials and pre-filled syringes. Leveraging the unique expertise and technological superiority developed by Meiji Seika Pharma, we will contribute to society by providing a stable supply of reliable products while enhancing our cost competitiveness.
Manufacture of solid preparations -> Manufacturing process for solid drugs
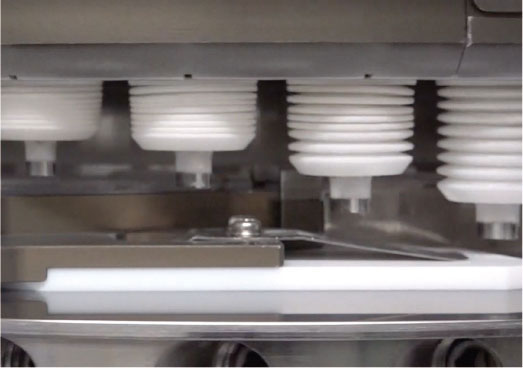
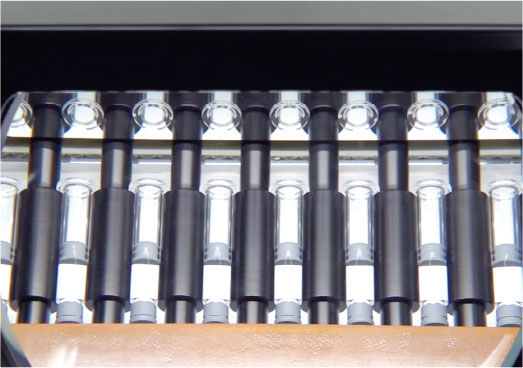
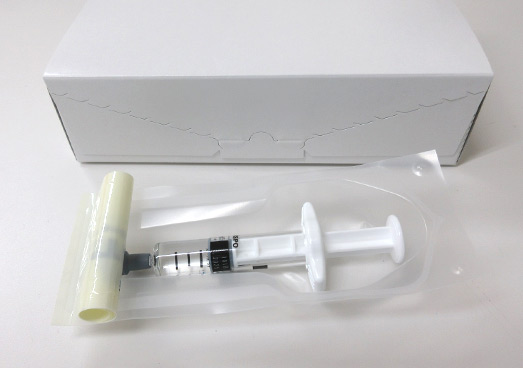
Manufacture of pre-filled syringes -> Manufacturing process for prefilled syringes
Quality Assuarance
We guarantee the quality of our products by ensuring they are manufactured according to methods approved by the pharmaceutical regulatory bodies and meet standards. We also conduct self-inspections of production activities in compliance with GMP, objectively review the status of manufacturing and quality control processes, and work to improve quality.