Dedication to Quality Assurance
Our comprehensive QC & QA services
Meiji Seika Pharma Group has established the following reliability policy
to conduct our business activities. As a member of the group, we strictly adhere to this policy.
Quality Assurance Policy
We conduct research, development, and reliable production of beneficial and high-quality products across various areas of pharmaceuticals. In addition, our commitment is to ensure the stable supply of products to patients and healthcare professionals, as well as to provide necessary
product-related information appropriately and rapidly.
Through these activities, we conduct our business with the objective of contributing to society.
Acting with sincerity and humility, all our employees will continue to work diligently every day so that patients and healthcare
professionals can use the products and information we provide with confidence.
We invite our business partners to understand our mission and collaborate with us.
To ensure the reliability of our products and information, we follow the guidelines outlined above as our business
activity policy.
By doing so, we will strive to earn the trust of patients and medical professionals and contribute to society.
According to the Meiji Seika Pharma Regulatory Compliance and Quality Assurance Guidelines, established based on the above-mentioned Regulatory Compliance and Quality Assurance Policy, we are committed to ensuring the reliability of our products with a strong sense of mission and pride.
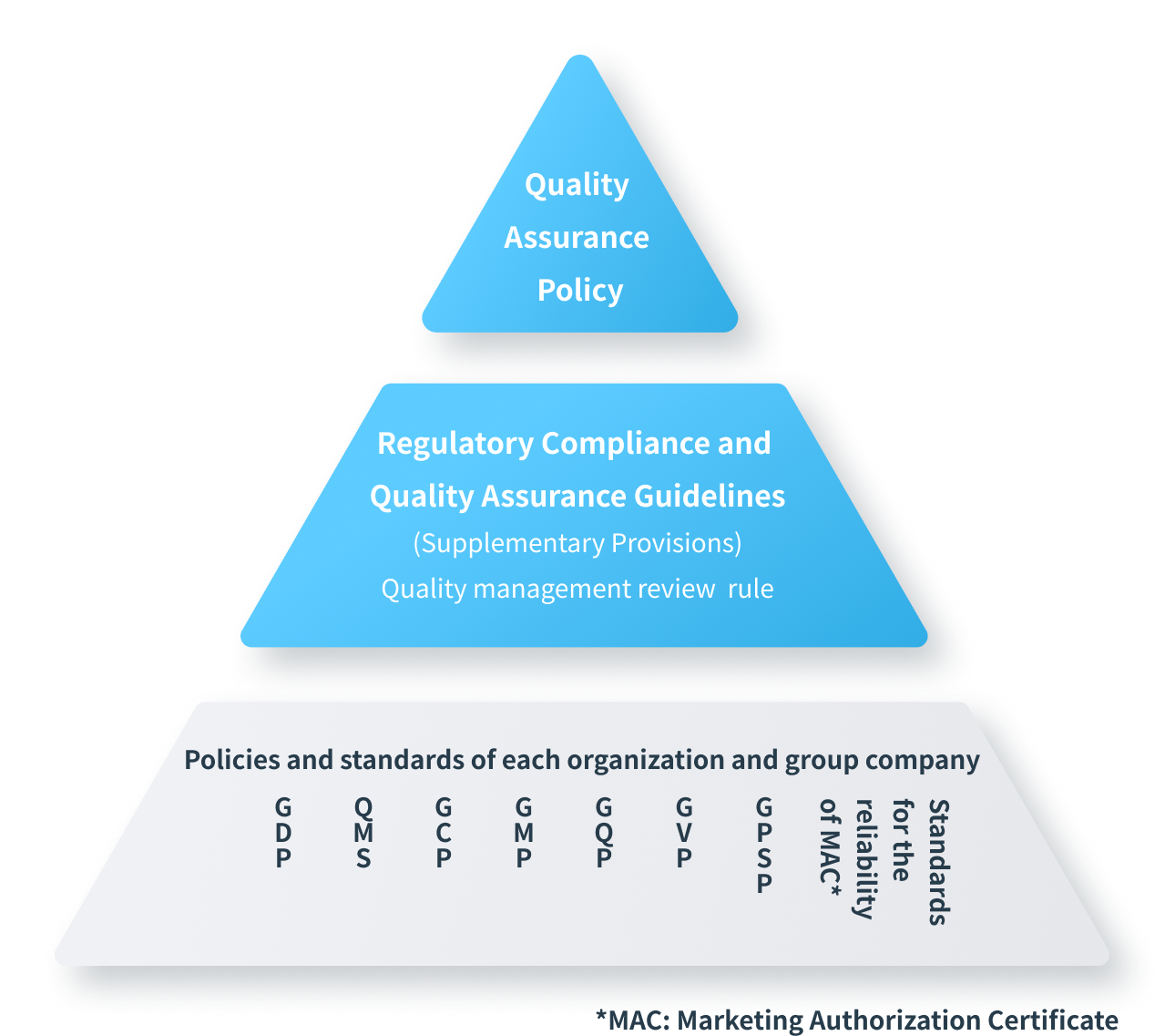
- GCP
- Good Clinical Practice
- GMP
- Good Manufacturing Practice
- GQP
- Good Quality Practice
- GVP
- Good Vigilance Practice
- GPSP
- Good Post-marketing Study Practice
- QMS
- Quality Management System
- GDP
- Good Distribution Practice
Our Approach to Quality Assurance
Establishment of a robust quality assurance system
In pharmaceutical quality assurance, it is essential requirement to manage documents beyond simple file storage. This includes clearly defining the author and approver, establishing the document's valid/invalid status, and ensuring that records are maintained over the long term
without any possibility of tampering.
For this reason, we have implemented a document management system to ensure the secure long-term storage of GMP and other regulated documents.
Furthermore, by digitizing processes such as OOS (Out-of-Specification) investigations,
abnormality/deviation handling, and change management, we achieve timely information sharing with all relevant departments.
We conduct consistency checks against the Marketing Authorization whenever the approved content is modified, thereby ensuring accurate verification of alignment.

Activities to establish a highly transparent quality culture
Over the decades, meiji has continued its efforts to cultivate a quality-oriented culture. We are instilling in all employees the importance of reporting deviations and appropriately handling them completely in accordance with GMP, while promoting a culture of quality compliance.
- Unannounced self-inspection
- To ensure comprehensive misconduct prevention, we are striving for an even higher standard of compliance. In addition to conducting self-inspections in compliance with laws and regulations, we perform them unannounced to ensure transparency and thoroughly reinforce quality compliance.
- On-site inspection by QA personnel
- By having our QA staff regularly present at the production sites, we can respond promptly and accurately to even minor issues that occur on-site and drive problem-solving.
In our daily production activities, we are actively strengthening quality compliance directly on the manufacturing floor.
Initiatives to deliver trusted quality to our customers
Based on scientific data, we analyze production performance from multiple perspectives and verify stable production, enabling us to prepare for high quality annual review reports and earn the confidence of our customers.
Has a track record of numerous regulatory inspections and customer audits

Compliance with Global Quality Standards
We possess extensive inspection experience, having successfully undergone inspections by the FDA, PMDA, and Chinese regulatory authorities, and conduct manufacturing and quality control in compliance with global regulations.
track record
- Japan
- America
- China
- Brazil
track record*
- Japan
- Thailand
- Indonesia
- China
- Vietnam
- South Korea
- Taiwan
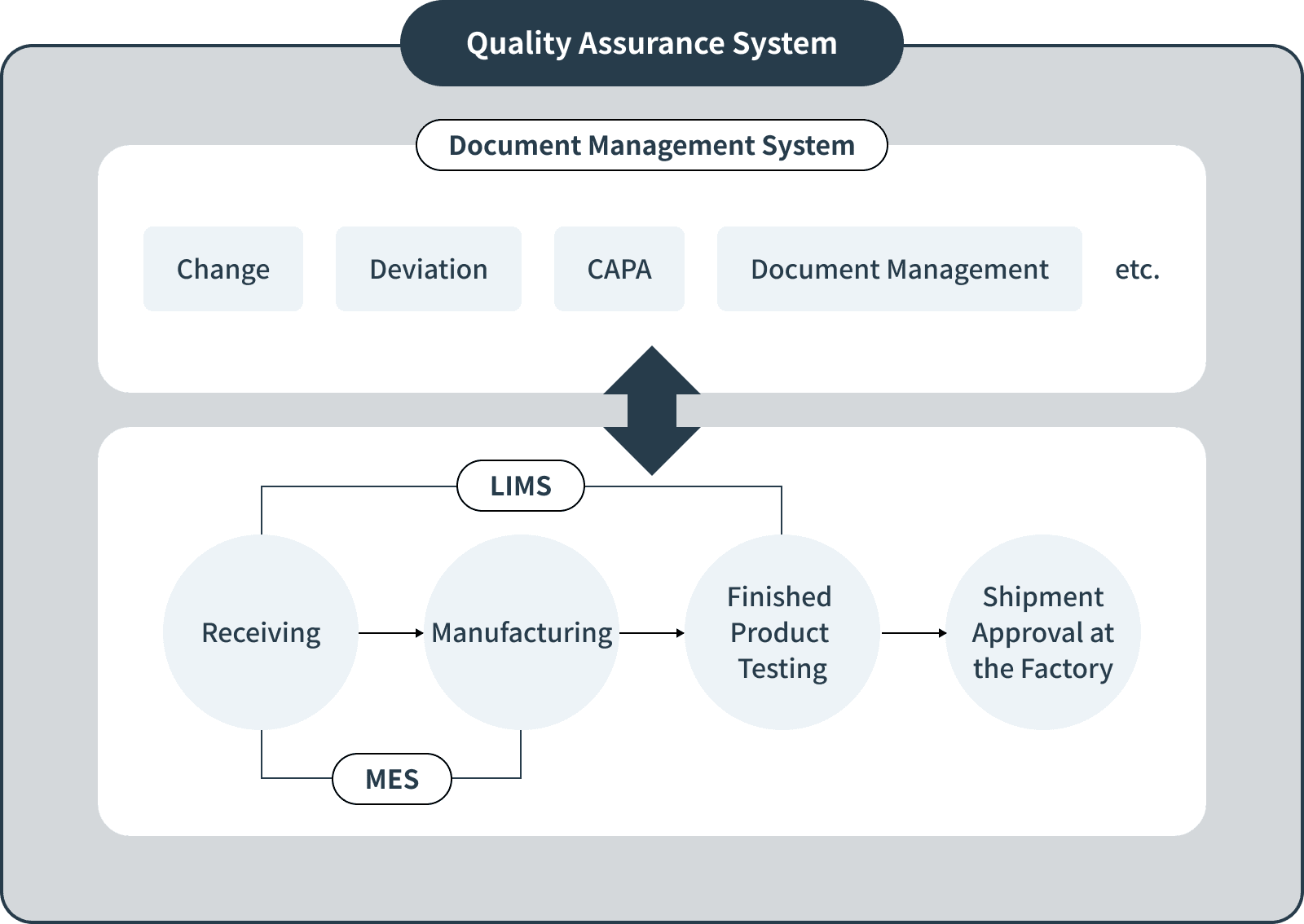
Quality Assurance System
We are working to improve the efficiency and ensure the reliability of manufacturing and quality control through digitized process management.
- Integrated control and traceability of manufacturing processes using MES (Manufacturing Execution System)
- Pursuing accuracy of testing data and efficiency of management using LIMS (Laboratory Information Management System)
- Implementing of a Document Management System to promote electronic document management for all quality-related activities
By making full use of these systems, we are focusing not only on improving production efficiency, but also on strengthening our quality control system and improving reliability.
We will continue our efforts and continuous improvement to maintain the highest quality.